Which of the Following Best Describes Ionic Bonding
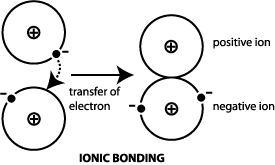
Which of the following best describes ionic bonding. 100 1 rating An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions.
Ces Information Guide Materials Science Engineering
Graphite has layered structureLayers are held by van der Waals forces and distance between two layers is 340 pm.

. Which of the following models best describes the bonding within a layer of the graphite structure. Calcium easily loses electrons and. Representative particle is a molecule.
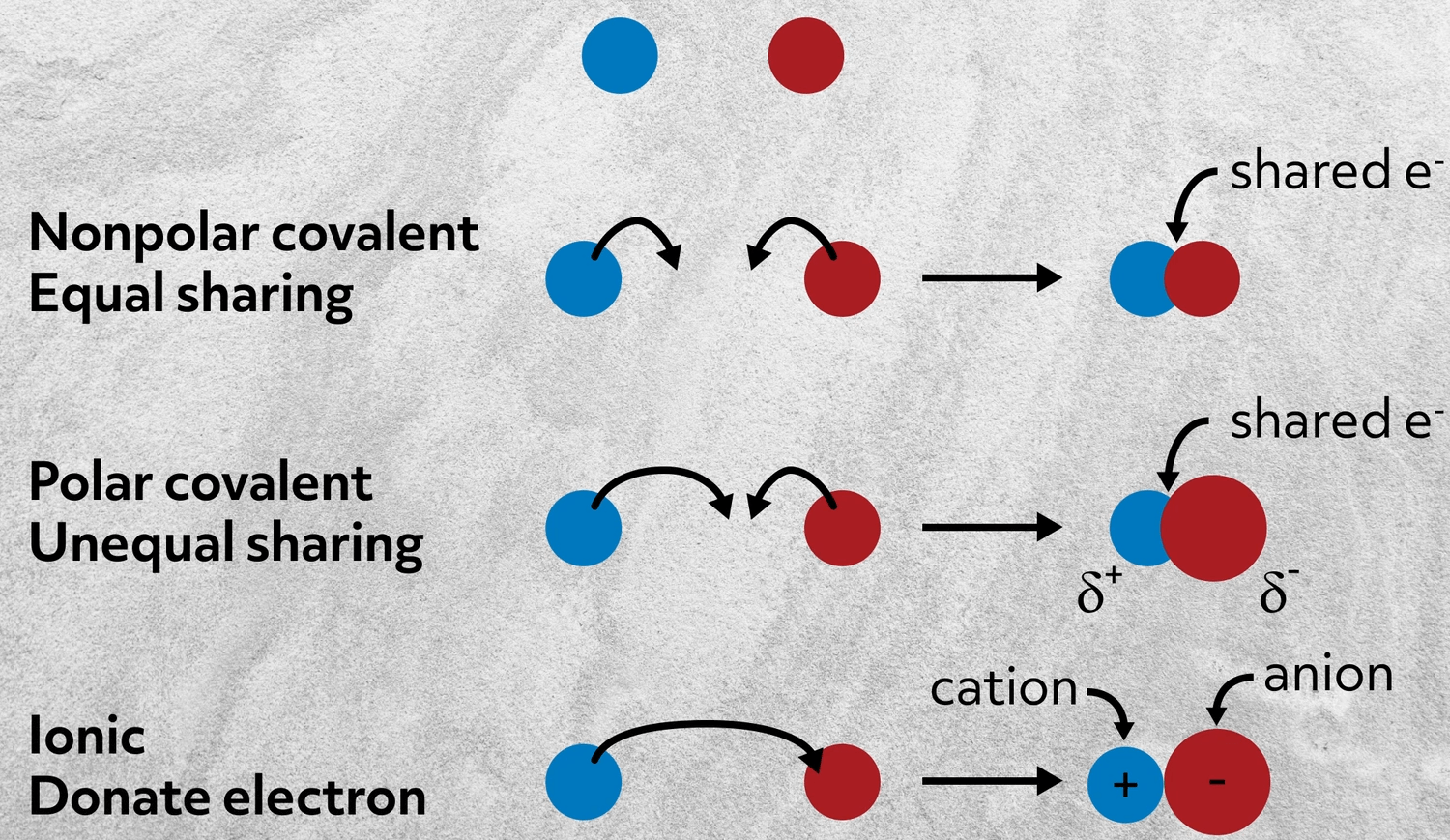
The attraction of ions due to the transfer of valence electrons D. Each layer is composed of planar hexagonal rings of carbon atomsC Cbond length with in the layer is 1415 pm Each carbon atomin hexagonal ring undergoes sp2 hybridisation and. The sharing of a pair of electrons between two atoms a relatively weak bond.
Which of the following statements best describes metallic bonding. The transfer of electrons from the metal to the non-metal. A aspartate b glycine c serine.
3 non-metallic covalent bonding. Strong forces of attraction between positively charged metal ions and. Phosphorus easily loses electrons and chlorine gains them.
The number of electrons lossed or gained by each ion. Which of the following models best describes the bonding within a layer of the graphite structure. Ionic bonds form so that the outermost energy level of atoms are filled.
Oxygen easily loses electrons and lithium gains them. Which type of chemical bond is formed by the electrostatic force between a positive ion and a negative ion. Hydrogen easily loses electrons and carbon gains them.
Which of the following best describes ionic bonding. A A compound that contains the element lead. Which of the following best describes ionic bonding.
A Metal transfers or donates its electron to a non-metal forming an ionic compound B. An ionic bond between oppositely charge ions. Which of the following statements best describes a lead compound.
N-F A I only B II only C III only D I and III E I II and III 3 Which of the following BEST describes the bonding found within solid Al 2 O 3. The transfer of electrons from the metal to the non-metal. Strong forces of attraction between positively charged metal ions and negatively charged.
Aone atom giving up some of its electrons to another atom bwhen two elements with same charge are held together by electrostatic forces ctwo atoms exchanging a set of electrons dtwo atoms. The attraction between two charged atoms a relatively weak bond in an aqueous solution. During chemical changes an ionic bonding forms when atom give away its electrons from another atom.
An ionic bond between oppositely charge ions. The transfer of valence electrons between atoms to make them neutral C. The sharing of valence electrons between two or more neutral atoms.
Non-metals share electrons to become stable forming a compound whose. So it is unsaturated ie. Though graphite is a metal layer of graphite has non-metallic bond as here bonding is the valence electrons to other empty valence orbital.
Non-metals share electrons to become stable forming a compound whose representative particle is a molecule C. A Strong covalent bonds between atoms with similar electronegativities. Ionic bonds is a bond that forms when electrons is being transferred from one form to another form.
2 Which of the following bonds would be best categorized as covalent. Identify which of the following amino acids has a side chain that may be important in binding a drug by ionic bonding. This is the best answer based on feedback and ratings.
Metal transfers or donates its electron to a non-metal forming an ionic. Which of the following statements best describes an ionic bond. Ionic bonds are fo.
All of the above. The repulsion of ions due to the transfer of valence electrons B. Which of the following models best describes the bonding class 11 chemistry CBSE.
A two atoms sharing a set of electrons B two atoms exchanging a set of electrons C one atom giving up some of its electrons to another atom D when two elements with same charge are held together by electrostatic forces. Which of the following statements best describes metallic bonding. The sharing of a pair of electrons between two atoms a relatively strong bond.
Chemical Bonding and Molecular Structure Covalent Bond Which of the following models best descr. Van der Waals forces. Here each carbon atom is attached with three other carbon atoms.
Which of the following best describes a pair of elements that will form an ionic bond. Which results in positive and negative ions. Which of the following best describes ionic bonding.

No comments for "Which of the Following Best Describes Ionic Bonding"
Post a Comment